However this modification can be through different metabolic pathways and does not necessarily mean a reduction in toxicity. Molecules that share the same chemical formula but differ in the placement structure of their atoms andor chemical bonds are known as isomers.
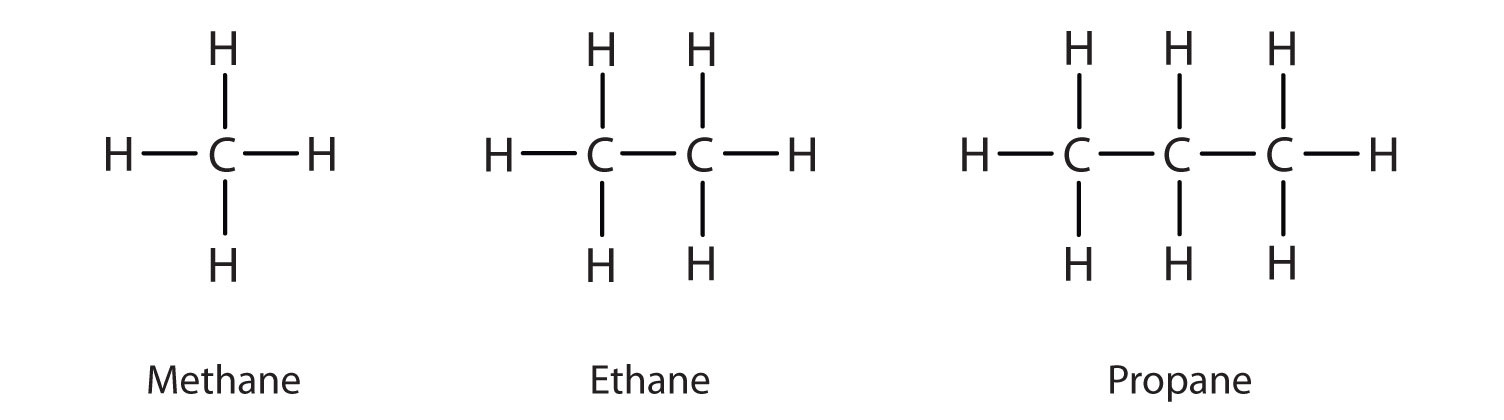
Structures And Names Of Alkanes
There are many such compounds which tend.
. Both molecules have four carbons and ten hydrogens C 4 H 10 but the different arrangement of the atoms within. Instead the EZ notation is used based on the priority of the substituents using the CahnIngoldPrelog CIP priority rules for absolute configuration. The formula for benzene the simplest arene and base structure for all others is C6H6.
For example the structure of capsaicin found in chili peppers incorporates several functional groups labeled in the figure below and explained throughout this section. As we progress in our study of organic chemistry it will become extremely important to be able to. Hydrocarbons are the principal constituents of petroleum and natural gas.
Structural isomers such as butane and isobutane differ in the placement of their covalent bonds. Any compound with a benzene ring is called an aromatic compound. Biodegradation is the biologically catalyzed modification of an organic chemicals structure.
The carbon atoms join together to form the framework of the compound and the hydrogen atoms attach to them in many different configurations. They serve as fuels. The IUPAC standard designations E and Z are unambiguous in all cases and therefore are especially useful for tri- and tetrasubstituted.
In Chapter 7 we noted that alkanessaturated hydrocarbonshave relatively few important chemical properties other than that they undergo combustion and react with halogensUnsaturated hydrocarbonshydrocarbons with double or. If a phospholipid is smeared over a small hole in a thin piece of plastic immersed in water a stable planar bilayer of phospholipid molecules is created at the hole. Because of the two pendant alkyl chains present in phospholipids and the unusual mixed charges in their head groups micelle formation is unfavorable relative to a bilayer structure.
As shown in the following. Most are made from petroleum. Our modern society is based to a large degree on the chemicals we discuss in this chapter.
Even if other parts of the molecule are quite different certain functional groups tend to react in certain ways. Mineralization one type of biodegradation is defined as the conversion of an organic substance to its inorganic constituents rendering the original. Hydrocarbon any of a class of organic chemical compounds composed only of the elements carbon C and hydrogen H.
Cistrans notation cannot be used for alkenes with more than two different substituents.
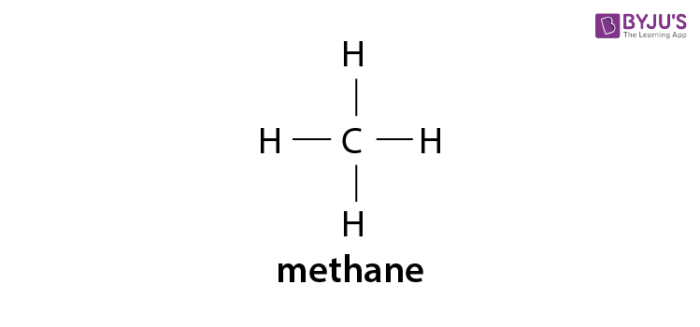
Alkanes Formula Definition Structure Properties List Of Alkanes Videos Examples And Faqs Of Alkanes
What Is The Bonding Structure Of Alkanes Quora
Organic Chemistry Structure Of Alkanes Introduction To Organic Molecules Sparknotes

0 Comments